15. Acidity of Amorphous Silica-Alumina Catalysts
First-principles calculations reveal the dominant acid sites on the amorphous silica-alumina (ASA) catalyst surface. Based on the strength of interaction with Lewis base probe molecules, the bridging and pseudo-bridging silanol (PBS) species are determined to be the most acidic and therefore catalytically active groups. Evident in the calculations is the interplay between the Lewis acid and the Brønsted acid sites on the ASA catalyst surface, giving rise to the enhanced acidity of the PBS species. This work provides mechanistic insight to inform efforts at rationally engineering enhanced ASA-based solid acids.
Keywords: catalysts, surfaces, amorphous silica-alumina (ASA), acidity, first-principles, computations
15.1. Background
Amorphous silica-alumina (ASA) substrates possess both Brønsted and Lewis acid sites making ASA well suited to be used as a support for multifunctional industrial catalysts. Determining the local chemistry of the acid sites on the surface of ASA is of critical interest to understand the basis for ASA activity and uncover structure-activity relationships. There are a number of chemically unique silanol species on the ASA surface and it was unknown which species were mainly responsible for ASA’s activity. First-principles calculations were carried out to determine the underlying mechanism and relative acidity of the various ASA surface silanol groups. [1]
15.2. Computed Results
Different surface silanols were considered, including:
- bridging silanol [O–Si–O(H)–Al]
- silanol bound to Al through the lattice [(H)O–Si–O–Al]
- pseudo-bridging silanol (PBS) configuration [O–Si–O(H)···Al/Si(IV) ]
To assess the relative acidity of these species, the chemical interactions with a series of Lewis base probe molecules (carbon monoxide, ammonia, pyridine, and lutidine) were evaluated. A comparison of adsorption energies and binding modes indicated that bridging silanol and PBS are the most acidic. Furthermore, as presented in Figure 15.2.1, the chemical behavior of PBS interacting with the probe molecules highlighted the active role the Lewis acid site plays in enhancing the Brønsted acidity of silanol. As seen from the O-Al distance across the probe molecule series, the interaction between the silanol oxygen and the framework Al site tracks the basicity of the probe molecule; with complete proton transfer leading to covalent O-Al bond formation. The Lewis acidity is converted to Brønsted acidity by increased stabilization of the Si-O- anion and adaptation of the cation coordination number to form a O-Al/Si linkage.
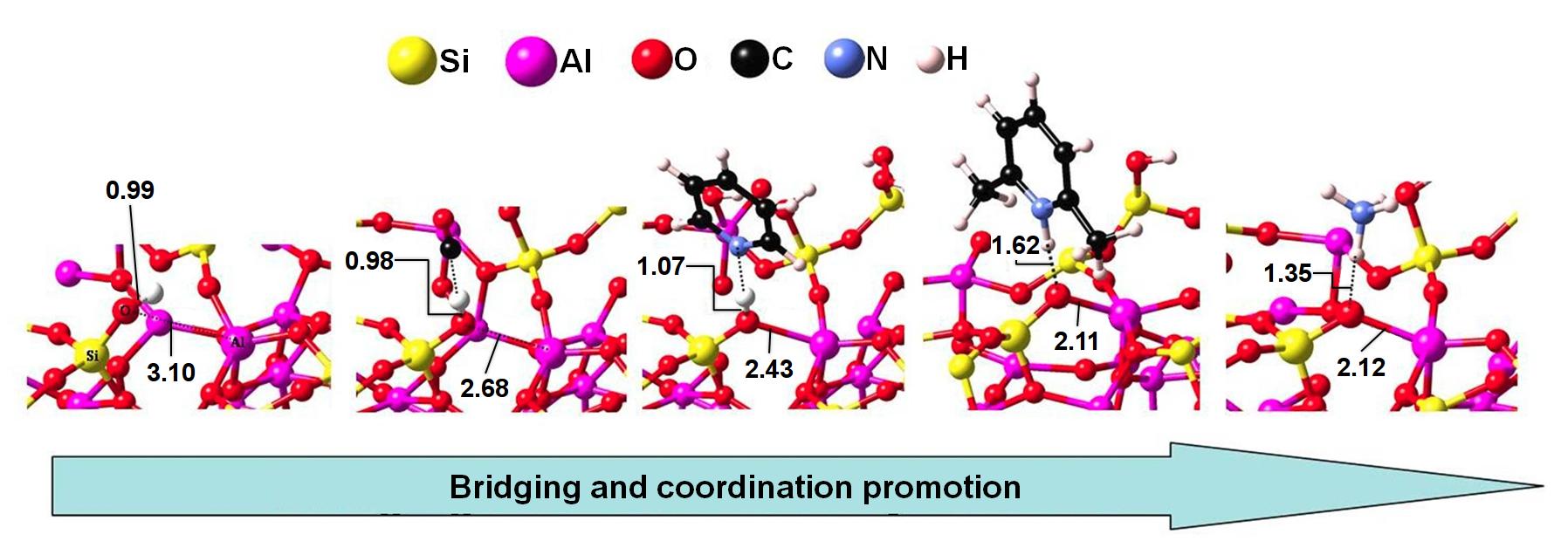
Figure 15.2.1 Adsorption structures for PBS-Al. Left to right: PBS-Al, carbon monoxide, pyridine, lutidine, and ammonia. (Adapted from [1])
15.3. Significance
Identifying the chemical species responsible for catalytic activity is critical to determine the structural characteristics required for highly active catalyst surfaces. Insight into the chemical mechanisms underlying catalysis can provide design rules for the development of improved catalysts. Given the widespread use of acidic ASA-supports in multifunctional catalyst applications, an in-depth understanding of ASA catalytic groups have a broad impact. The interplay between Lewis acid and Brønsted acid sites on the ASA surface provides guidance in the design of solid acids with enhanced activity.
First-principles calculations are a reliable source for predicting the atomistic structure, adsorption energetics and the underlying physical and chemical interactions for catalytic surfaces as demonstrated here for the case of ASA substrates. With the rapidly increasing performance of computational resources, the fundamental properties controlling the catalytic activity for increasingly complex systems such as nanostructures can be predicted with high confidence, thus guiding the design of highly efficient and selective industrial catalysts.
Required MedeA Modules
- MedeA Environment
- MedeA JobServer and TaskServer
- MedeA Pearson
- MedeA VASP
| [1] | (1, 2) C. Chizallet, and P. Raybaud, “Acidity of Amorphous Silica-Alumina: From Coordination Promotion of Lewis Sites to Proton Transfer”, Chem. Phys. Chem. 11, 105 (2010). DOI |
| download: | pdf |
|---|