22. Low-Strain Cathode Materials for Solid-State Li-Ion Batteries
The volume change of active materials that accompanies charge and discharge of Li-ion batteries is a major source of degradation, which limits the overall lifetime of such a battery [1]. While a zero-strain anode material exists, namely Li4Ti5O12[2], no suitable zero- or low-strain cathode material is known so far. By using systematic DFT calculations, three low-strain materials have been identified within the class of LiMn2-y-zCryMgzO4. The most promising materials have been synthesized and characterized by X-ray diffraction and electrochemical techniques. The results are consistent with the ab initio predictions [3], [4].
Keywords: Li-ion batteries, cathode materials, low-strain cathodes, transition-metal oxides
22.1. Background
Long-term stability and safety are indispensable requirements in the search for new battery materials with higher capacities. While the need for safer batteries has initiated the development of solid-state electrolytes, new challenges emerged regarding the mechanical stability at the interfaces with the electrodes. Since most of the known active materials used as negative and positive electrodes experience expansion and contraction during operation, cracks will form at the interfaces with the rigid electrolyte causing degradation of the battery performance [1]. Whereas on the anode side Li4Ti5O12 has been shown to operate with nearly zero strain [2], a cathode material with similar properties was still missing at the time the present study was initiated. Candidate electrode materials as given in Figure 22.1.1 showed either rather low voltage or large volume change as in the case of the previous benchmark material LiNi0.5Mn1.5O4[5]. The present application note describes a set of ab initio calculations as based on density functional theory (DFT), which were performed to investigate a class of transition-metal oxides based on the spinel structure [3], [4].
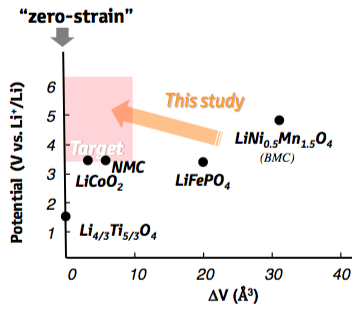
Figure 22.1.1 Li chemical potentials and volume changes of candidate cathode materials. The shaded area marks the target materials with a much lower volume change than the previous benchmark material LiNi0.5Mn1.5O4.
22.2. Method of Calculation
The DFT calculations were performed using MedeA®[6] VASP [7], which uses projector-augmented (PAW) potentials and wave functions [8]. Exchange and correlation effects were included within the semilocal GGA as parametrized by the PBEsol scheme [9]. In a first step, DFT calculations were used to evaluate the volume change of the spinel-type compounds LixM2O4, where M = Mg, Al, V, Cr, Mn, Fe, Co, Ni, Cu, on lithiation and delithiation. To this end, three different values for the lithium content were considered, namely, x = 0, 0.5, 1.0. All structures were fully relaxed and different spin-structures were taken into account. In addition, deviations from the cubic symmetry underlying the spinel-structure were allowed for. In a second step, the previous results were used to minimize the volume change of the quasi-ternary compounds LiM12-y-zM2yM3zO4in an approach closely following Vegard’s law [10].
22.3. Computed Results
The results of the ab initio calculations for the pure spinel-type compounds are summarized in Figure 22.3.1, which shows for each compound the calculated volume at the three Li concentrations mentioned above. Note that at this stage the fact that some of these compounds may not even exist in nature was ignored. The lines included in Figure 22.3.1 were obtained from parabolic fits to the data points. Obviously, all transition metals induce a volume increase with increasing lithium concentration, whereas only Al and Mg cause a decrease of the cell volume, which in case of Mg is particularly strong. As a consequence, any quasi-ternary spinel-type compound must contain a non-negligible fraction of Mg to counterbalance the volume increase of the transition-metal spinels on lithiation. Hence, Mg was the first choice to be inserted into the basic cathode material LiMn2O4. Yet, since Mg is electrochemically inactive, Cr was chosen as a third partner because of the many oxidation states offered by this element.
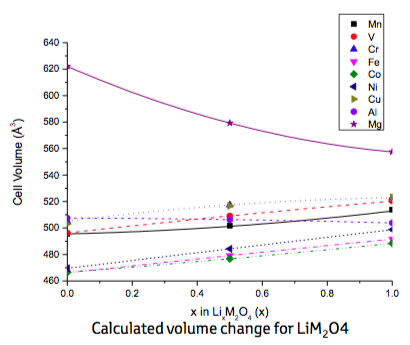
Figure 22.3.1 Calculated volume change for base spinel constituents LiM2O4(M = Mg, Al, V, Cr, Mn, Fe, Co, Ni, Cu).
After these preparations the next step consisted of optimizing the relative concentrations at the metal sites of the quasi-ternary compound LiMn2-y-zCryMgzO4in order to minimize the volume change on lithiation and delithiation. To this end, three different approaches were used, namely, (1) full unconstrained optimization to obtain zero-strain behavior, (2) constrained optimization to find a low-strain material with Mn content 2-y-z larger than 1 in order to ensure structural stability, and (3) constrained optimization to find a low-strain material with Mg content z smaller than 0.2 in order to reduce the amount of highly oxidized Cr necessary to achieve full charge. The results of these three approaches together with the volume change of the benchmark compounds LiNi0.5Mn1.5O4are displayed in Figure 22.3.2. As expected, unconstrained optimization indeed leads to zero volume change, however, at a very small Mn and big Cr content. More interesting are the two low-strain configurations LiMn1.1Cr0.5Mg0.4O4, and LiMn0.59Cr1.21Mg0.2O4with volume changes of 0.6% (3 Å3) and 1.6% (8 Å3), respectively, much lower than the value of 6% (30 Å3) reported for LiNi0.5Mn1.5O4.
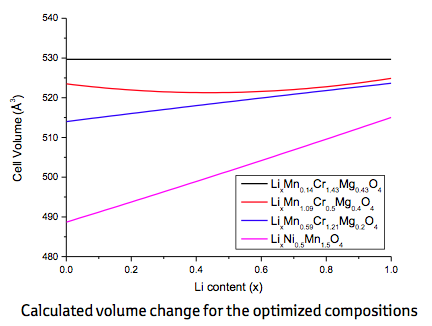
Figure 22.3.2 Calculated volume change for the optimized spinel compositions LiMn0.14Cr1.43Mg0.43O4, LiMn1.1Cr0.5Mg0.4O4, and LiMn0.59Cr1.21Mg0.2O4. The volume change for the benchmark LiNi0.5Mn1.5O4is also shown.
In order to confirm the validity of the approach and to make a close connection to existing experimental data, the previous optimizations were complemented with calculations of the volume changes of a set of selected compositions, namely, LiNi0.5Mn1.5O4, LiCu0.25Ni0.25Mn1.5O4, LiCr0.5Mn1.5O4, LiFe0.5Mn1.5O4, LiCo0.16Mn1.84O4, and LiAl0.15Mn1.85O4, which were found in very good agreement with measured values in all cases [4].
Powder samples of all three optimized compounds were synthesized at Toyota Motors Europe [3]. However, XRD patterns of the two samples with high Cr concentration revealed the presence of at least two phases, indicating a phase separation induced by the high Cr content. The instability caused by high Cr content is consistent with the fact that LiCr2O4does not exist. In contrast, LiMn1.1Cr0.5Mg0.4O4could be synthesized in almost pure form and was thus used to build electrochemical cells in order to investigate the volume change upon charging and discharging the cells. As a result, ex situ XRD patterns showed a volume change of 4 Å in very good agreement with the calculations. In addition, charge curves taken for this system revealed a performance comparable to that of the benchmark material LiNi0.5Mn1.5O4.
The DFT calculations also provided detailed insight into the mechanisms resulting in a near zero-strain behavior. A synergistic compensation mechanism underlies the desired property as illustrated in Figure 22.3.3, which includes the bond lengths calculated for an eightfold supercell of LixMn1.125Cr0.5Mg0.375O4. With increasing Li concentration, the Mg-O bonds tend to decrease, the Mn-O bonds remain similar, while the Cr-O bonds tend to increase. As a result, the overall volume of the crystal structure changes little upon charging and discharging with Li ions. Furthermore, the microscopic analysis thus well reflects the behavior of the “pure” compounds as displayed in Figure 22.1.1. This behavior is also in close analogy to observation for the zero-strain mechanism for Li4Ti5O12, where local distortions in the crystal structure likewise allow this material to keep the volume nearly unchanged upon lithium insertion [11].
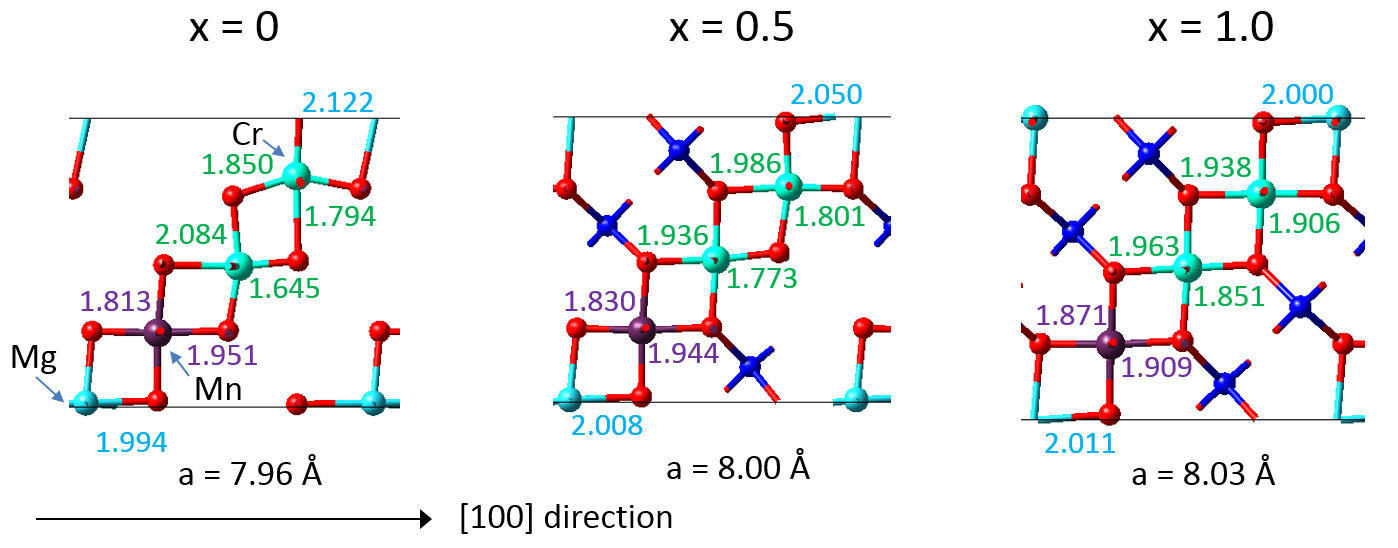
Figure 22.3.3 Calculated interatomic distances of LixMn1.125Cr0.5Mg0.375O4for increasing Li concentration.
22.4. Conclusion
In this project, DFT simulations have been combined with an optimization approach based on Vegard’s law to obtain a spinel-type quasi-ternary compound with small volume change on lithiation and delithiation. The procedure has been confirmed by comparison to measured volume changes obtained for various compositions. In addition, experiments performed for the best candidate, LiMn1.1Cr0.5Mg0.4O4, found a volume change of 0.8% (4 Å3) in very good agreement with the calculated 0.6% (3 Å3). Finally, analysis of the DFT results allowed to understand the observed volume changes from a microscopic compensation mechanism.
22.5. Acknowledgment
This application note is based on published work performed as a contract research project for Toyota Motors Europe. We are especially grateful to Dr. F. Rosciano of Toyota Motors Europe for initiating this project, for proposing to focus on spinel-type compounds with Mn as a transition metal, and for carrying out the synthesis and characterization of the proposed compounds.
MedeA modules used in this application
- MedeA Environment
- MedeA VASP
| [1] | (1, 2) M. Ebner, F. Marone, M. Stampanoni, and V. Wood, “Visualization and Quantification of Electrochemical and Mechanical Degradation of Li Ion Batteries”, Science 342, 716 (2013) (DOI) |
| [2] | (1, 2) T. Ohzuku, A. Ueda, and N. Yamamoto, “Zero-Strain Insertion Material of Li[Li1/3Ti5/3] O4for Rechargeable Lithium Cells”, J. Electrochem. Soc. 142, 1431 (1995) (DOI) |
| [3] | (1, 2, 3) F. Rosciano, M. Christensen, V. Eyert, A. Mavromaras, and E. Wimmer, “Reduced Strain Cathode Materials for Solid State Lithium Ion Batteries”, International Patent WO2014191018, 4 December 2014 (DOI) |
| [4] | (1, 2, 3) F. Rosciano, M. Christensen, V. Eyert, A. Mavromaras, and E. Wimmer, “Computational Design and Experimental Verification of Zero- and Low-strain Cathode Materials for Solid-State Li-Ion Batteries”, International Battery Association, Brisbane 2014 |
| [5] | M. Kundaraci and G. G. Amatucci, “Synthesis and Characterization of Nanostructured 4.7 V LixMn1.5Ni0.5O4Spinels for High-Power Lithium-Ion Batteries”, J. Electrochem. Soc. 153, A1345 (2006) (DOI) |
| [6] | MedeA and Materials Design are registered trademarks of Materials Design, Inc. |
| [7] | G. Kresse and J. Furthmüller, “Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set”, Phys. Rev. B 54, 11169 (1996) (DOI); “Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set”, Comput. Mater. Sci. 6, 15 (1996) (DOI) |
| [8] | P. E. Blöchl, “Projector augmented-wave method”, Phys. Rev. B 50, 17953 (1994) (DOI). |
| [9] | J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. Zhou, and K. Burke, “Restoring the Density-Gradient Expansion for Exchange in Solids and Surface”, Phys. Rev. Lett. 100, 136406 (2008) (DOI); “Perdew et al. Reply”, Phys. Rev. Lett. 101, 239702 (2008) (DOI); “Erratum: Restoring the Density-Gradient Expansion for Exchange in Solids and Surface [Phys. Rev. Lett. 100, 136406 (2008)]”, Phys. Rev. Lett. 102, 039902 (2009) (DOI) |
| [10] | L. Z. Vegard, “Die Konstitution der Mischkristalle und die Raumfüllung der Atome”, Z. Phys. 5, 17 (1921) (DOI) |
| [11] | K. Ariyoshi, R. Yamato, and T. Ohzuku, “Zero-strain insertion mechanism of Li[Li1/3Ti5/3] O4for advanced lithium-ion (shuttlecock) batteries”, Electrochim. Acta 51, 1125 (2005) (DOI) |
| Revision: | 8032 |
|---|---|
| download: | pdf |