MedeA Transition State Search - Maps Out Reaction and Diffusion Pathways, Locates Transition States, and Calculates Activation Barriers
Transitions states are key for describing the kinetics and rates of activated processes, such as chemical reactions and mass transport in solids, interfaces, and on surfaces.
At-a-Glance
MedeA® [1] Transition State Search (TSS) maps out transition paths, i.e. how atoms are rearranged in chemical reactions and diffusion processes, and determines the structure and energy of transition states. Combined with MedeA Phonon, this allows you to compute reaction rates and jump rates as a function of temperature.
Key Benefits
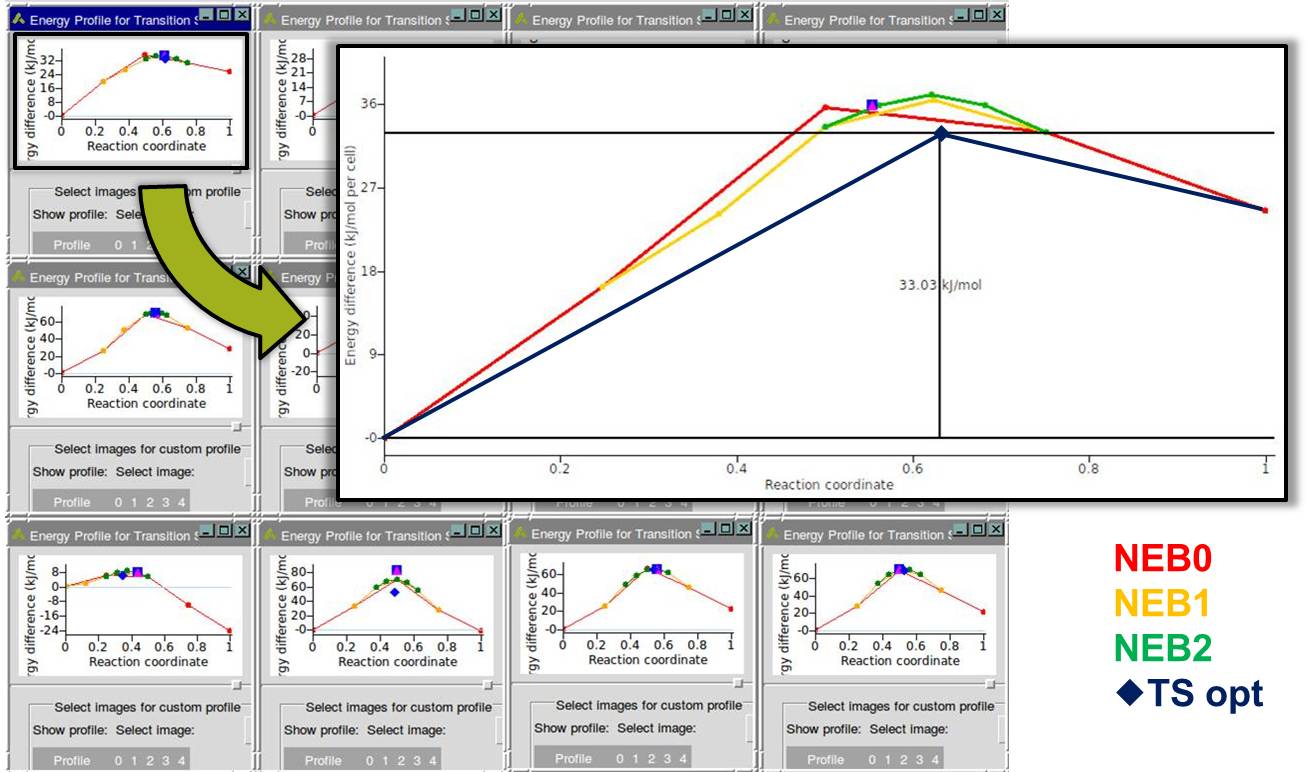
- Fully automated multi-step computational procedure search transition paths, and optimize transition states in a single run
- Unrestricted and flexible parallelization of calculations
- Several different computational techniques can be combined or separately applied
- Customizable energy profile visualization and structural animation of over-barrier motion
Transition states determine important system properties, such as reaction rates and activation energy barriers, which govern kinetic and diffusive behavior. Since transition states are located at saddle points of the potential energy surface representing local maxima, along one particular direction, special algorithms are required to locate and refine associated structures. MedeA Transition State Search makes the location of such transition states computationally efficient.
MedeA Transition State Search can be used to find transition states and activation energies, for surface reactions such as the dissociation of a di-sulfide bridge of a lubricant additive on the Cu2O (111) surface in the presence of hydroxyl groups. The blue line in the figure below connects the energy minima with the refined maximum, representing the transition state.

With combinations of different computational techniques, MedeA Transition State Search finds energy barriers of a multitude of diffusion pathways of atoms in solids, exemplified in the figure below for migration of Li cations in a battery cathode material.
‘I was also interested in formulating the path of chemical reactions.’ -Kenishi Fukui
Properties from MedeA Transition State Search
- Structure and energy of transition states of chemical reactions, and for diffusion of atomic or molecular species
- Energy profile along reaction or diffusion path
- Structures and energies of multiple minima and transition states
- Structural animation of reaction/diffusion path
The figure below shows the calculated diffusivity of hydrogen in fcc Ni compared to experiments [2], and illustrates the level of accuracy which can be obtained with MedeA Transition State Search in combination with MedeA Phonon, MedeA VASP, and activated transition state theory as described in [2]. The computed diffusion coefficient (solid line) as a function of temperature is in excellent agreement with measurements (individual points), even for tiny isotope anomalies shown in the insert.

Computational Characteristics
- Automatic setup of initial transition path by linear interpolation or manually specified custom structures
- Map out minimum energy path with the Nudged Elastic Band (NEB) method [3] or its double nudged variant [4]
- Multiple automatic refinements of the path, i.e. automatic zoom on the region near the transition states, providing a new set of NEB images
- Further refine the transition state candidates by
- Final optimization of transition states, using a gradient minimizer (quasi Newton approach)
- Search for transition state in a specific direction, following the lowest eigenmode by means of the Dimer method
- Transition state exploration in randomly chosen directions by the Dimer method
- Global BFGS optimizer for the NEB approaches
- Fully integrated with the MedeA JobServer and TaskServer infrastructure
- Efficient employment of computational resources in locating transition states
Required Modules
- MedeA Environment
- MedeA Transition State Search
- MedeA VASP
Recommended Modules
- MedeA Phonon
- MedeA Forcefield Optimizer
- MedeA Diffusion
Find Out More
Learn how to apply MedeA Transition State Search in the following Materials Design Application Notes:
- Adsorption and Dissociation of Iodine Molecules on a Zr Surface
- Catalytic Isomerization of pent-2-ene in H-ZSM-22
- Diffusion of Hydrogen in Nickel
| [1] | MedeA and Materials Design are registered trademarks of Materials Design, Inc. |
| [2] | (1, 2) E. Wimmer, W. Wolf, J. Sticht, P. Saxe, R. Najafabadi, and G.A. Young Jr, Physical Review B 77, 134305 (2008) |
| [3] | H. Jonsson, G. Mills, K.W. Jacobsen, in Classical and Quantum Dynamics in Condensed Phase Simulations Eds. BJ. Berne, G. Ciccotti and D.F. Coker, p. 385 (World Scientific, 1998) |
| [4] | S. A. Trygubenko and D. J. Wales, J. Chem. Phys. 120, 2082 (2004). |
| [5] | G. Henkelman, B. Uberuaga, H. Jonsson, Journal of Chemical Physics 113, 9901 (2000) |
| [6] | R. A. Olsen, G. J. Kroes, G. Henkelman, A. Arnaldsson, and H. Jonsson, J. Chem. Phys. 121, 9776 (2004) |
| download: | pdf |
|---|